I am so mad right now. I was pointed to this website by someone who knows I like my coffee less than scalding:
I am not mad at Dave or Dave, but at myself. I’m an engineer. I hate “too hot” coffee and am not enthralled by cold coffee. I know about thermodynamics. And yet, did it occur to me to do something like this? No. I’d like to think I look for problems and solutions, but I completely whiffed on this. I blame my god-given lack of inspiration, creativity and entrepreneurship.
What do these do and how do they do it?
Well, in a perfect world, our coffee maintains a constant (hot) temperature for as long as we care to drink it. A Goldilocks temperature. Not too hot, not too cold. However, according to thermodynamics, thermal energy moves from high to low temperatures. That is, assuming you’re not drinking your coffee in a very hot sauna, it will relentlessly transfer heat to your surroundings (via radiation, convection, and conduction) until it has assumed the same temperature as its surroundings.
There is a small caveat to this; at the point of a phase change, the temperature of a substance will remain the same even as it transfers heat. For example, a well-mixed glass of ice water at a uniform temperature of zero degrees Celsius will absorb heat while remaining at zero degrees until all the ice is melted. So H2O can be zero degrees as a liquid and a solid; the liquid phase just contains far more heat energy than the ice phase.
Knowing the temperature they consider ideal (say 140 F), our friends Dave and Dave exploited this phase change property to maintain a temperature for as long as the mug can inhibit heat loss. They impregnated each Joulie with a material that changes phase from solid to liquid at 140 F.
Adding too-hot coffee to the mug will heat up the Joulies to 140 F at which point they will start to melt (absorbing heat all the time). Assuming you’ve used enough Joulies in your coffee, the insides shouldn’t quite melt all the way before cooling the coffee off to 140 F (or else the coffee would “cool” to some temperature above 140 F). At this instant, when the coffee is the same temperature as the melting point of the interior of the Joulies, there will be no heat transfer between the two.
Oh yeah, added bonus! Because heat transfer depends on the difference in temperature, by initially cooling the coffee to a drinkable temperature, the Joulies actually reduce the initial degree of heat loss to the surrounding environment!
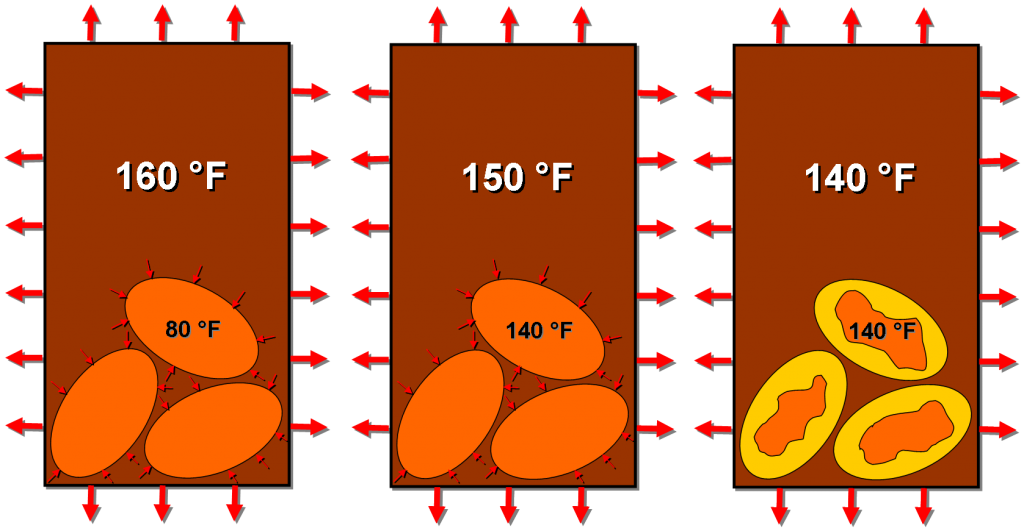
Heat is absorbed by the Joulies (causing the orange solid phase to melt into the gold liquid phase) until the coffee and Joulies are the same temperature
Our Goldilocks temperature coffee, however, will continue to lose heat to the environment. But, once the Joulies equilibrate with the coffee at 140 F, they will maintain that temperature as they release heat into the coffee (due to the liquidy bits solidifying).
NOTE: Just to make a point about heat transfer moving from high to low temperatures, I show the coffee as 139 F; it can actually be infinitely close to 140 F, it just has to be a smidgen lower than the Joulies in order to permit heat transfer. The actual temperature difference and uniformity will depend on harder to quantify things, like the degree of mixing in the coffee and the heat transfer coefficients between all of our materials.
Once the internals of the Joulies have completely solidified, they can now cool off to temperatures below 140 F, and so, of course, will the coffee until it reaches temperatures more appropriate for a hot tub than for drinking. But this unfortunate, inevitable conclusion will be greatly delayed, allowing a longer window to enjoy your tasty beverage!
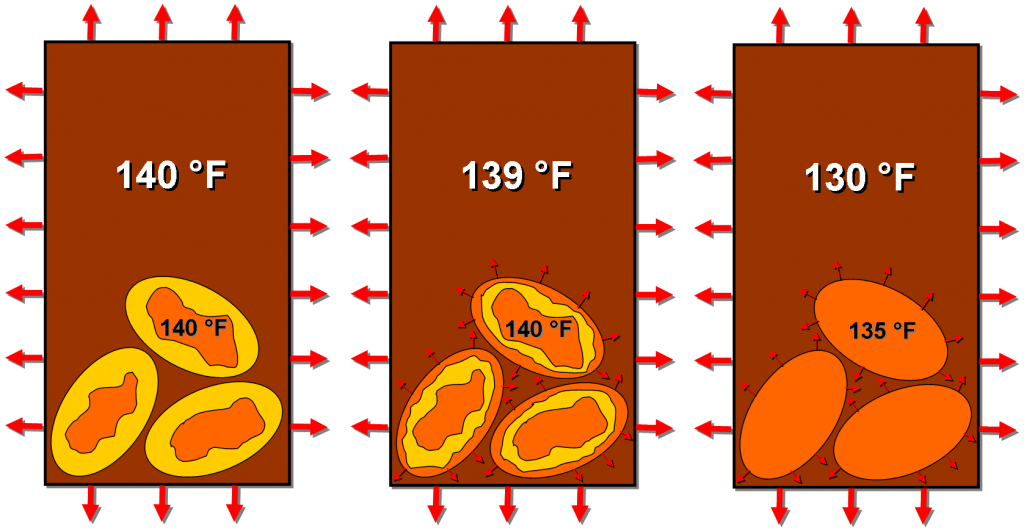
Once the internals have melted and assumed the same temperature as the coffee, the Joulies can release stored heat back into the coffee, maintaining a more or less constant temperature
So again, kudos to Dave and Dave. Finding a simple, robust solution to an actual (if not life-and-death) problem. This is engineering at its best.


“A Goldilocks temperature”. Ha Ha Ha! Great idea. Great post.
The they said internal product inside is all natural. What is the product?
What makes it stay @ 140 what about 180 ?
Thanks
Bob
Last I checked, they don’t specify what it is, saying it is proprietary…
How about using this process to replace utility costs? Hot water heaters? Heating homes???
Hm. I think the biggest obstacle would be finding the right substance that is both cheap and safe that would experience a phase change at a desirable temperature (say 72 degrees Fahrenheit). Nothing comes to mind…
[…] into your coffee. If you would like a scientific explanation on how this invention works, this is a great article that explains the heat transfer of the Coffee Joulies.They claim that your coffee will cool down to the optimal temperature 3 times faster then normal (by […]